Amplite® Cholesterol Quantitation Kit
Cholesterol is required to build and maintain cell membranes. It modulates membrane fluidity over the range of physiological temperatures. Within cells, cholesterol is the precursor molecule in several biochemical pathways. Cholesterol is also an important precursor molecule for the synthesis of Vitamin D and the steroid hormones, including the adrenal gland hormones cortisol and aldosterone as well as the sex hormones progesterone, estrogens, together with testosterone and their derivatives. This Amplite® Cholesterol Quantitation Assay Kit provides one of the most sensitive methods for quantifying cholesterol. The kit uses Amplite® Red to quantify the concentration of cholesterol, which is related to the production of hydrogen peroxide in the cholesterol oxidase-mediated enzyme coupling reactions in the presence of cholesterol. The amount of cholesterol is proportional to the concentration of hydrogen peroxide formed in the enzyme coupling reaction cycle. In the presence of peroxidase, the fluorescence intensity of Amplite® Red is proportional to the concentration of hydrogen peroxide that is converted to the concentration of cholesterol. The assay can be readily read with a fluorescence microplate reader.
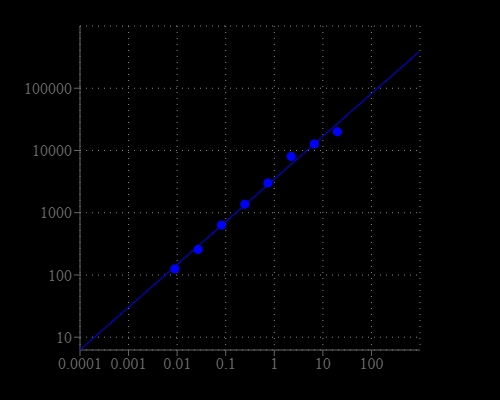

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 40006 | 200 Tests | Price |
Spectral properties
| Excitation (nm) | 571 |
| Emission (nm) | 584 |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 13, 2026

