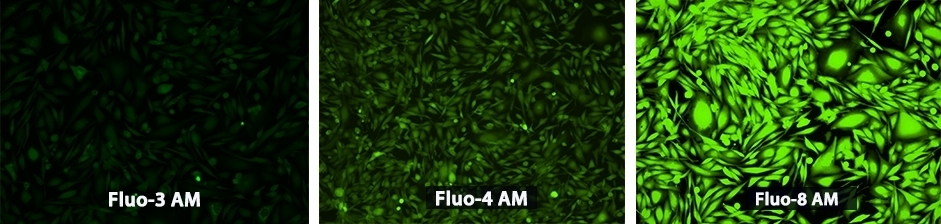
Fluo-8®, AM
Example protocol
PREPARATION OF STOCK SOLUTIONS
Unless otherwise noted, all unused stock solutions should be divided into single-use aliquots and stored at -20 °C after preparation. Avoid repeated freeze-thaw cycles
Prepare a 2 to 5 mM stock solution of Fluo-8® AM in high-quality, anhydrous DMSO.
PREPARATION OF WORKING SOLUTION
On the day of the experiment, either dissolve Fluo-8® AM in DMSO or thaw an aliquot of the indicator stock solution to room temperature.
Prepare a 2 to 20 µM Fluo-8® AM working solution in a buffer of your choice (e.g., Hanks and Hepes buffer) with 0.04% Pluronic® F-127. For most cell lines, Fluo-8® AM at a final concentration of 4-5 μM is recommended. The exact concentration of indicators required for cell loading must be determined empirically.
Note: The nonionic detergent Pluronic® F-127 is sometimes used to increase the aqueous solubility of Fluo-8® AM. A variety of Pluronic® F-127 solutions can be purchased from AAT Bioquest.
Note: If your cells contain organic anion-transporters, probenecid (1-2 mM) may be added to the dye working solution (final in well concentration will be 0.5-1 mM) to reduce leakage of the de-esterified indicators. A variety of ReadiUse™ Probenecid products, including water-soluble, sodium salt, and stabilized solutions, can be purchased from AAT Bioquest.
SAMPLE EXPERIMENTAL PROTOCOL
Following is our recommended protocol for loading AM esters into live cells. This protocol only provides a guideline and should be modified according to your specific needs.
- Prepare cells in growth medium overnight.
On the next day, add 1X Fluo-8® AM working solution to your cell plate.
Note: If your compound(s) interfere with the serum, replace the growth medium with fresh HHBS buffer before dye-loading.
Incubate the dye-loaded plate in a cell incubator at 37 °C for 30 to 60 minutes.
Note: Incubating the dye for longer than 2 hours can improve signal intensities in certain cell lines.
- Replace the dye working solution with HHBS or buffer of your choice (containing an anion transporter inhibitor, such as 1 mM probenecid, if applicable) to remove any excess probes.
- Add the stimulant as desired and simultaneously measure fluorescence using either a fluorescence microscope equipped with a FITC filter set or a fluorescence plate reader containing a programmable liquid handling system such as an FDSS, FLIPR, or FlexStation, at 490/525 nm cutoff 515 nm.
Spectrum
Product family
| Name | Excitation (nm) | Emission (nm) | Extinction coefficient (cm -1 M -1) | Quantum yield |
| Fluo-8FF™, AM | 495 | 516 | 23430 | 0.161 |
| Fluo-8H™, AM | 495 | 516 | 23430 | 0.161 |
| Fluo-8L™, AM | 495 | 516 | 23430 | 0.161 |
| Fluo-3, AM *Bulk package* *CAS 121714-22-5* | 506 | 515 | 86,0001 | 0.151 |
| Fluo-3, AM *CAS 121714-22-5* | 506 | 515 | 86,0001 | 0.151 |
| Fluo-3, AM *UltraPure grade* *CAS 121714-22-5* | 506 | 515 | 86,0001 | 0.151 |
| Fluo-4 AM *Ultrapure Grade* *CAS 273221-67-3* | 495 | 528 | 82000 | 0.161 |
| Fluo-5F, AM *Cell permeant* | 494 | 516 | - | - |
| Fluo-5N, AM *Cell permeant* | 494 | 516 | - | - |
Show More (1) | ||||
Citations
Authors: Imamura, Keiko and Nagahashi, Ayako and Okusa, Aya and Sakasai, Tomoki and Tsukita, Kayoko and Kutoku, Yumiko and Ohsawa, Yutaka and Sunada, Yoshihide and Sahara, Naruhiko and Kanaan, Nicholas M and others,
Journal: European Journal of Cell Biology (2025): 151484
Authors: Rabesahala de Meritens, Camille and Carreras-Sureda, Amado and Rosa, Nicolas and Pick, Robert and Scheiermann, Christoph and Demaurex, Nicolas
Journal: Journal of Cell Biology (2025)
Authors: Lee, Jee Woong and Lee, Junhee and Lee, Jungha and Kim, Duhee and Hong, Woongki and Lee, Junghyup and Song, Minyoung and Kang, Hongki
Journal: Advanced Materials Interfaces (2025): 2400873
Authors: You, Wenting
Journal: (2025)
Authors: Liu, Anqi and Peng, Peng and Wei, Changze and Meng, Fanhui and Huang, Xiaoyao and Liu, Peisheng and Fan, Siyuan and Cai, Xinyue and Wu, Meiling and Xuan, Zilin and others,
Journal: Advanced Science (2025): 2407446
References
Authors: Bednar B, Cunningham ME, Kiss L, Cheng G, McCauley JA, Liverton NJ, Koblan KS.
Journal: J Neurosci Methods (2004): 247
Authors: Martin VV, Beierlein M, Morgan JL, Rothe A, Gee KR.
Journal: Cell Calcium (2004): 509
Authors: Cheng H, Song LS, Shirokova N, Gonzalez A, Lakatta EG, Rios E, Stern MD.
Journal: Biophys J (1999): 606
Authors: do Ceu Monteiro M, Sansonetty F, Goncalves MJ, O'Connor JE.
Journal: Cytometry (1999): 302
Authors: Su ZL, Li N, Sun YR, Yang J, Wang IM, Jiang SC.
Journal: Shi Yan Sheng Wu Xue Bao (1998): 323





![Relationship of Ca<sup>2+</sup> dynamics with electrical stimulation frequency. Relative changes in fluorescence intensity ((ΔF/F0)×100%) for leg muscle of (A) beetle orally dosed with Fluo-8 (blue) and Cell Tracker (red) and (B) control beetle measured with the filter setting used for Fluo-8 (blue) and Cell Tracker (red) under varying electrical stimulations (1 Hz, 10 Hz, 50 Hz, and 100 Hz; 10% duty cycle; 2 V). Data were analyzed from the ROI adjacent to the stimulated site (S1B Fig.). The error bars represent the S.D. (N = 8 beetles, n = 24 beetle legs for (A); N = 2 beetles, n = 8 beetle legs for (B)). The small numbers next to each plot indicate the order of stimulation. Cell Tracker data set was compared with Fluo-8 data set at each stimulation frequency evaluated by student’s t-test both for dosed beetles in (A) (1st 50 Hz, p = 9.37×10-4; 10 Hz, p = 7.45×10-3; 1st 1 Hz, p = 2.30×10-1; 100 Hz, p = 4.17×10-4; 2nd 1 Hz, p = 4.49×10-2; and 2nd 50 Hz, p = 8.16×10-3) and for control beetles in (B) (1st 50 Hz, p = 4.10×10-1; 10 Hz, p = 9.30×10-1; 1st 1 Hz, p = 9.29×10-1; 100 Hz, p = 5.69×10-2; 2nd 1 Hz, p = 6.85×10-1; and 2nd 50 Hz, p = 2.67×10-2). The significant differences are displayed by an asterisk (p < 0.05). Fluo-8 intensity dynamics show that Ca<sup>2+</sup> dynamics inside the muscle have a positive correlation with electrical stimulation frequency; i.e., higher stimulation frequency induces larger increase in [Ca<sup>2+</sup>]. On the other hand, Cell Tracker intensity dynamics show that the frequency-dependent intensity change due to muscle displacement is not apparent. Source: Graph from <strong>Oral Dosing of Chemical Indicators for <em>In Vivo</em> Monitoring of Ca<sup>2+</sup> Dynamics in Insect Muscle</strong> by Ferdinandus et al., <em>PLOS</em>, Jan. 2015.](/_next/image?url=https%3A%2F%2Fimages.aatbio.com%2Fproducts%2Ffigures-and-data%2Ffluo-8-am%2Ffigure-for-fluo-8-am_EQOEG.jpg&w=128&q=25)
