Amplite® Luminometric Nitroreductase Assay Kit
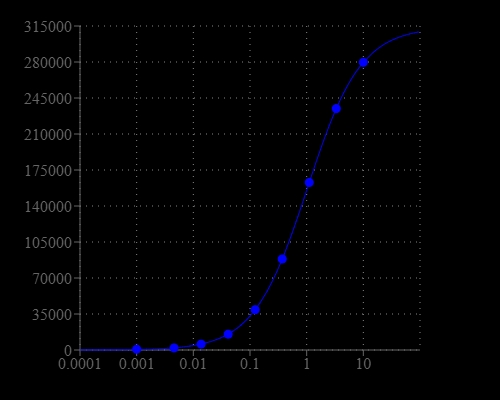
Nitroreductases (NTR) are a family of evolutionarily related proteins involved in the reduction of nitrogen-containing compounds. They are absent in mammalian cells but widespread in bacteria. Nitroreductases play a crucial role in the reduction of nitroaromatic compounds via NAD(P)H-dependent reactions. NTR are also targets for developing novel antibiotics, degradation of pollutants, and cancer therapy. Although the quantification of NTR becomes extremely important for a number of biological applications, most of the current NTR assays are based on ELISA format with low sensitivity and low throughput. Amplite®™ Luminometric Nitroreductase Assay Kit provides a highly sensitive and convenient method to quantify NTR activity. The kit uses a luciferin derivative that can be selectively reduced by NTR luciferin. The amount of generated luciferin is conveniently detected with the well-known luciferase detection system. The luminescent intensity generated is proportional to the NTR activity. This luminometric nitroreductase assay kit has been formulated to have minimal hands-on time and stable luminescence signal. It can detect as low as 20 ng/mL of NTR.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 12470 | 100 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Luminescence microplate reader | |
| Recommended plate | Solid white |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 19, 2026
