Amplite® Fluorimetric Glutathione Peroxidase Assay Kit
Blue Fluorescence
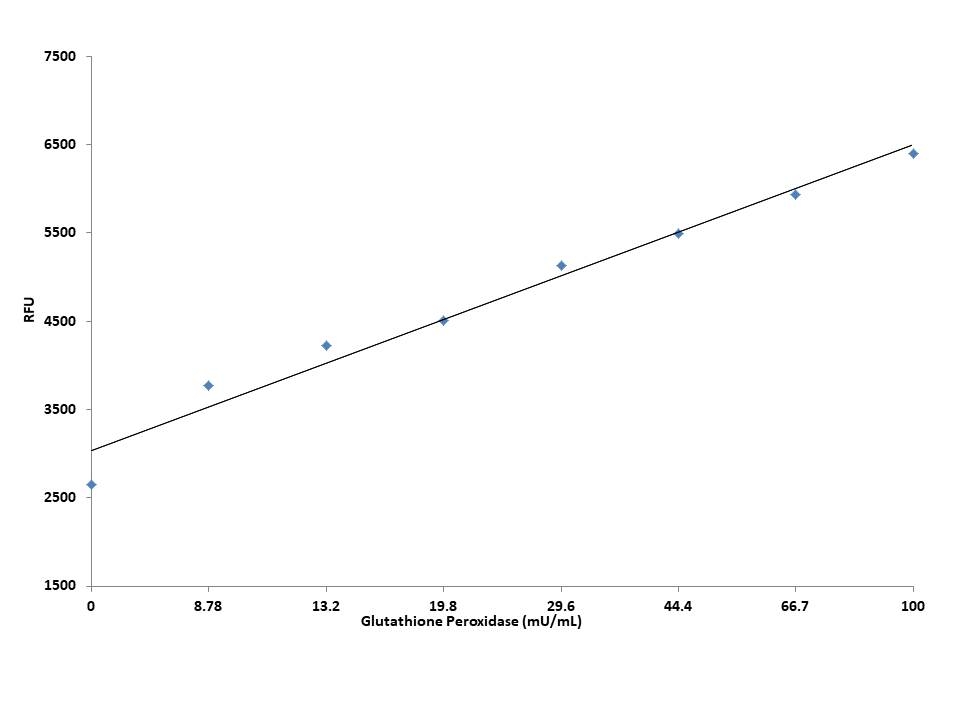
Glutathione peroxidase is an enzyme family with peroxidase activity to protect the organism from oxidative damage. Glutathione peroxidase plays an important role in reducing organic hydroperoxides such as lipid hydroperoxides to their corresponding alcohols, or reducing free hydrogen peroxide to water. Glutathione peroxidase guards against oxidative damage to cell membranes and other oxidant-sensitive sites in cells. The altered glutathione peroxidase levels correlate with lesions caused by many common and complex diseases. Glutathione peroxidase level is measured in biological samples as a potential indicator for the potential treatment of cancer, diabetes, neurodegenerative and cardiovascular diseases. AAT Bioquest's Fluorimetric Glutathione Peroxidase Assay Kit offers a sensitive fluorimetric assay for measuring glutathione peroxidase levels in biological samples. This assay is based on the oxidation of glutathione (GSH) to oxidized glutathione (GSSG) catalyzed by glutathione peroxidase. The generated GSSG is recycled to its reduced state GSH by glutathione reductase (GR) and NADPH to generate NADP+ that can be specifically monitored using Quest Fluor™ NADP Probe, our newly developed proprietary NADP sensor. The NADP sensor reacts only with NADP to generate a fluorescent product. The fluorescence signal can be measured with a fluorescence microplate reader and is directly proportional to the glutathione peroxidase activity. Compared to other commercial kits that measure the decrease in absorbance of NADPH at 340 nm, our Quest Fluor™ NADP Probe can be used for quantify NADP level directly. With this fluorimetric glutathione peroxidase assay, as low as 1.25 mU/mL glutathione peroxidase can be detected in a 155 µL reaction volume.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 11560 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 420 nm |
| Emission | 480 nm |
| Cutoff | 430 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 31, 2026
