Amplite® Fluorimetric Glutamic Acid Assay Kit
Red Fluorescence
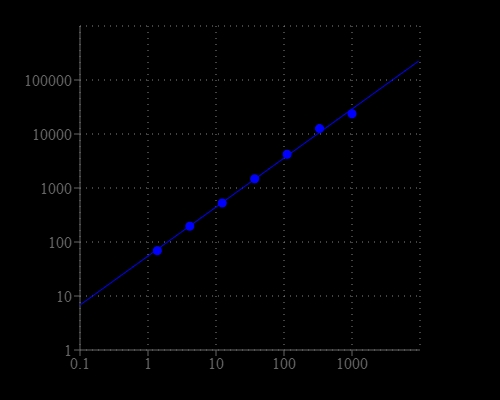
Glutamic acid is one of the 20 proteinogenic amino acids. The carboxylate anions and salts of glutamic acid are known as glutamates. Glutamate is an important neurotransmitter which plays a key role in long-term potentiation and is important for learning and memory. Glutamic acid is the precursor of GABA but has somewhat the opposite function. It might play a role in the normal function of the heart and the prostate. As one of the few nutrients that crosses the blood-brain barrier, glutamic acid is used in the treatment of diseases such as depression, ADD and ADHD, fatigue, alcoholism, epilepsy, muscular dystrophy, mental retardation, and schizophrenia. The Amplite® Fluorimetric Glutamic Acid Assay Kit provides a quick and sensitive method for the measurement of glutamic acid in various biological samples. In the assay, the coupled enzyme system catalyzes the reaction between L-glutamic acid and NADP to produce NADPH, which is specifically recognized by our NADPH sensor and recycled back to NADP. A red flurescence product is produced during the reaction. The signal can be read by either a fluorescence microplate reader or an absorbance microplate reader. With our Amplite® Fluorimetric Glutamic Acid Kit, we have detected as little as 10 µM glutamic acid in a 100 µL reaction volume. The assay is robust, and can be readily adapted for a wide variety of applications that require the measurement of glutamic acid.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 10054 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Fluorescence microplate reader | |
| Excitation | 540 nm |
| Emission | 590 nm |
| Cutoff | 570 nm |
| Recommended plate | Solid black |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 17, 2026
