Amplite® Colorimetric Glucose-6-Phosphate Assay Kit
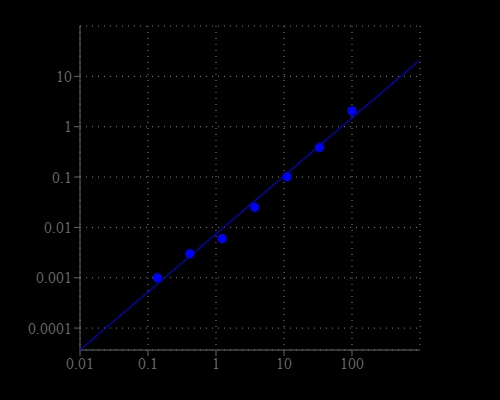
Glucose-6-phosphate (G6P) is a key intermediate for glucose transport into cells. G6P may also be converted to glycogen or starch for storage in the liver and muscles. G6P is utilized by glucose-6-phosphate dehydrogenase (G6PD) to generate the reducing equivalents in the form of NADPH. This is particularly important in red blood cells where a G6PDH deficiency leads to hemolytic anemia. AAT Bioquest's Amplite® Colorimetric Glucose-6-Phosphate Assay Kit provides a simple, sensitive and rapid method for detecting G6P in biological samples such as serum, plasma, urine, as well as in cell culture samples. In the coupled enzyme assay, the G6P concentration is proportionally related to NADPH that is specifically monitored by a chromogenic NADPH sensor. The absorption signal can be read by an absorption microplate reader at an absorbance ratio of A575 nm to A605 nm. With the Amplite® G6P Assay Kit, we were able to detect as little as 1 µM G6P in a 100 µL reaction volume.

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 13805 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12352200 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 575/605 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 29, 2026
