Amplite® Colorimetric Beta-Lactamase Activity Assay Kit
β-Lactamases are a large family of enzymes capable of hydrolyzing β-lactams. β-Lactam ring is the common element in all beta-lactam antibiotics including penicillin derivatives, cephalosporins, monobactams, and carbapenems. Through hydrolysis, β-lactamase breaks the β-lactam ring open, thus deactivates the molecule's antibacterial properties. Bacteria from clinical and non-clinical settings are becoming increasingly resistant to β-lactam antibiotics by synthesizing β-lactamase. To overcome this resistance, β-lactam antibiotics are often given with β-lactamase inhibitors such as clavulanic acid. Therefore, detection of β-lactamase activity is of central importance to assess beta-lactam antibiotics as well as to prevent antibiotics resistance. AAT Bioquest's Colorimetric Beta-Lactamase Activity Assay Kit offers a sensitive colorimetric assay for measuring β-lactamase activity in biological samples. The β-lactamase activity is detected using Nitrocefin, which changes color from yellow to red upon hydrolysis by β-lactamase. The assay can be performed using an absorbance microplate reader by measuring the OD ratio at the wavelength of 490 nm to 380 nm.
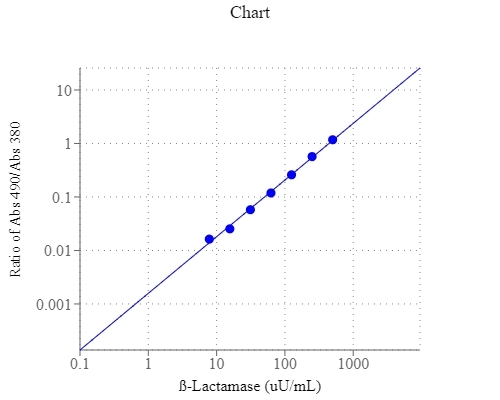

| Catalog | Size | Price | Quantity |
|---|---|---|---|
| 12551 | 200 Tests | Price |
Storage, safety and handling
| H-phrase | H303, H313, H333 |
| Hazard symbol | XN |
| Intended use | Research Use Only (RUO) |
| R-phrase | R20, R21, R22 |
| UNSPSC | 12171501 |
Instrument settings
| Absorbance microplate reader | |
| Absorbance | 490/380 nm |
| Recommended plate | Clear bottom |
Contact us
| Telephone | |
| Fax | |
| sales@aatbio.com | |
| International | See distributors |
| Bulk request | Inquire |
| Custom size | Inquire |
| Technical Support | Contact us |
| Request quotation | Request |
| Purchase order | Send to sales@aatbio.com |
| Shipping | Standard overnight for United States, inquire for international |
Page updated on January 24, 2026
